Artificial Disc Replacement Surgery: Cervical & Lumbar
Artificial Disc Replacement Surgery in Pune
Artificial disc replacement (ADR), also known as total disc replacement (TDR), falls under the category of arthroplasty. This surgical procedure involves the replacement of degenerated intervertebral discs within the spinal column with artificial devices, either in the lumbar (lower) or cervical (upper) spine. This procedure is used to treat persistent and intense lower back pain or cervical discomfort arising from degenerative disc disease.
Artificial discs are US FDA approved for placement in the cervical and lumbar region. This procedure is usually done in young patients with one or two level disease and the most important benefit of this procedure after removal of the whole disc and decompression of nerve roots is, it preserve the full range of motion between the two vertebral bodies.
Cervical Disc Replacement:
Only two discs are US FDA approved:
- Prestige LP by Medtronics
- Mobi-C by Zimmer Biomet
It is mainly indicated in young patients with 1 or 2 level of disc disease. After initial conservative management, surgery is advised. Post-surgery there is an improvement in the radicular pain and have minimum chances of adjacent level disc disease as compared with ACDF. Patient can be discharged early, need not to wear the cervical collar and can return back to work quickly. Full range of motion is preserved.
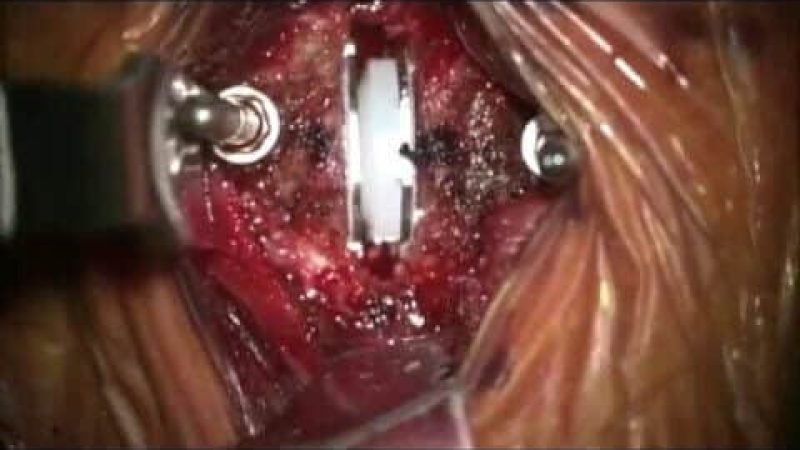
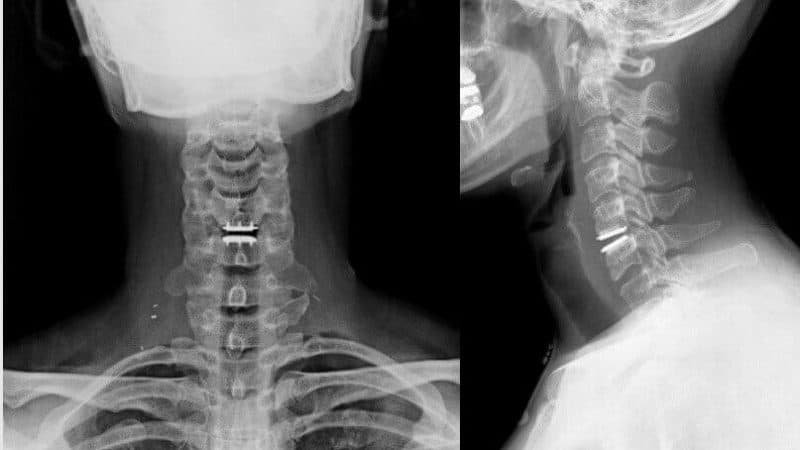
Cervical artificial disc replacement is contraindicated in:
- Older patients more than 67 years
- Infection
- Metal Allery
- Traumatic injury
- Osteoporotic bones
- Prior cervical fusion
- More than 2 levels of disc disease
Artificial Lumber Disc:
Lumbar artificial disc replacement is only approved for the single level.
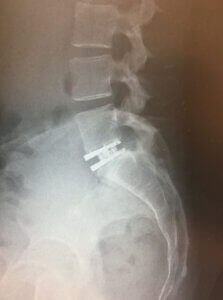
Dr. Vishal Bhasme performs Disc replacement surgery in Pune which is a type of spinal surgery used to treat specific spine conditions. It is important to discuss the potential risks and benefits of the surgery with a qualified spine surgeon to make an informed decision.